Transcript
Demyelination and axonal damage in CIDP
Claudia Sommer, MD
All transcripts are created from interview footage and directly reflect the content of the interview at the time. The content is that of the speakers and is not adjusted by Medthority.
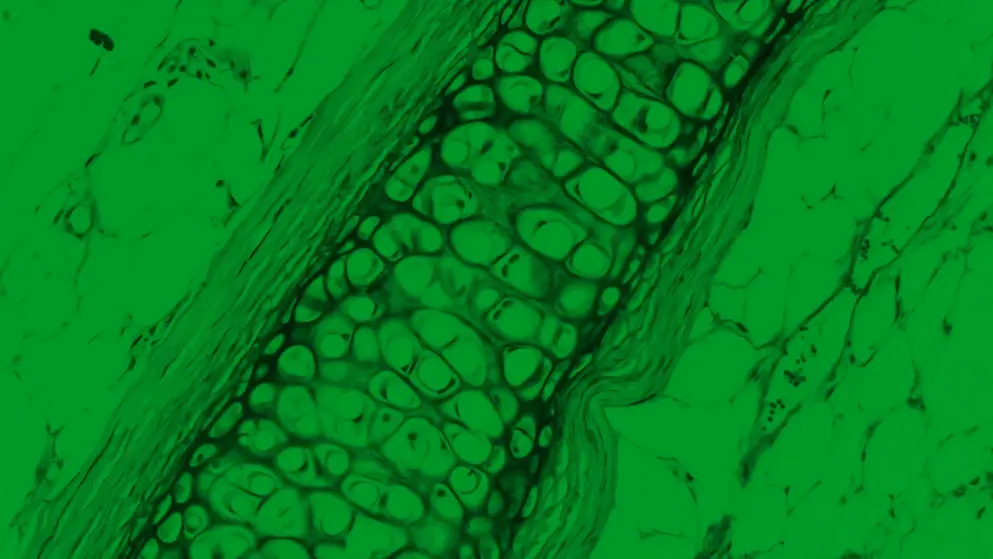
- You have all seen multiple cartoons of CIDP pathogenesis, and they tend to get more complicated over time. And usually, what is written above them or below them is a sentence like this one: that there is a complex interplay between multiple apparent immune responses, creating a pro-inflammatory environment, causing myelin and axonal damage. And this doesn't tell us in detail what happens. We know the players. We know that T cells are involved, B cells are involved, macrophages and complement, and the membrane attack complex. And how do we know this? We partially know this from old biopsy studies. Maybe some of you heard the talk by my colleague Katherine Doppler showing us what in the past the neuropathologists got out of sural nerve biopsies and what we learned from them. So we know that histopathological changes in CIDP include breakdown of the blood-nerve barrier. We know that there is segmental demyelination. We know this from the beautiful teased fibre studies that have been done. The Mayo Clinic was a pioneer in that. And we also know that there are various degrees of axonal damage. So for years and years, there has been a debate: Are humoral factors more important in CIDP, or are cellular factors more important? At the moment, we can only say both humoral and cellular macrophage-mediated demyelination play a crucial role in the pathophysiology of CIDP. Macrophage infiltration has indeed been shown in the myelinating fibres around the nodes of Ranvier.
We have shown them around the small blood vessels in the endoneurium, and they have been shown in the internodal region, and it seems that these macrophages are actively doing damage. And the complement system is a mediator in this system and plays a role in promoting both the demyelination and the axonal damage. And we also have evidence for this, because again, in these sural nerve biopsies from patients, complement deposition has been seen, and there are data showing increased complement activation in CIDP. So if we concentrate on the humoral factors, we think there are some autoantibodies, or at least IgG is involved. As you know, we do not have one autoantibody in CIDP, but IgG is involved and plays a role when this interplay of the antigen-presenting cells and T-cells comes into play, and in the end, complement is activated. So what is the evidence for the role of humoral factors? Some patients respond very beautifully to plasmapheresis, and they may respond very quickly to plasmapheresis. So this speaks for a circulating factor that we take out by this therapy, and then remyelination can happen and conduction blocks can go away. Also, as I mentioned, complement-fixing IgG and IgM deposits have been shown morphologically on myelin sheath of sural nerve biopsy samples.
And then, in experimental animals, some studies have shown that if you inject serum or purified IgG from CIDP patients into experimental animals, you can induce conduction block, which is usually considered a very important piece of information and speaking for this theory. So how could complement be involved in the CIDP pathophysiology? We could distinguish primary and secondary axonal damage. For example, we can envision that there are antibodies that we don't know yet exactly, binding to some perinodal proteins. These are complement-fixing antibodies. They might bind to C3b, and through the cascade, then the membrane attack complex becomes active and creates pores in the axonal membrane, which is detrimental to the axon and leads to axonal damage. Or another mechanism might be that there is primarily demyelination, but then the axon is demyelinated, it doesn't have protection anymore, and then the membrane attack complex might come into play. And in this scenario, the complement-fixing antibodies would be closer to the myelin.
These two processes do not need to exclude each other. They might happen in parallel. So there might be binding to the myelin sheath with complement activation, and then destruction of the myelin, and in parallel, the attack to the axon. So probably it's most likely that both processes happen and that there is an interdependence of the axonal damage and the demyelination in CIDP. And indeed, coming back to one of my favourite subjects, nerve biopsies, the biopsies from nerves from people with CIDP indeed show a lot of axonal loss and active axonal degeneration. So we think that the activated complement system via the membrane attack complex contributes to this axonal damage either directly or secondary to demyelination, or a combination of both. And what is important, we often talk of secondary axonal damage in our patients when they have deficits that don't seem to go away with treatment when they have been ill for a very long time. But recent evidence supports that axonal damage occurs very early in the disease. So maybe we should think of it earlier in the disease and in the treatment.
And again, this is a repetition. We don't know exactly what is primarily there, what is secondary, but these two processes are there in parallel. Let's look at the evidence for this statement that axonal damage occurs early in the disease. Even in the first descriptions of CIDP, where the images were shown of the nerves, there was a significant presence of axonal lesions. And this axonal damage predicts long-term disability more than the measures of demyelination. And we all know that our electrophysiological criteria for CIDP are based on demyelination, and for good reason. This is all based on evidence in the literature. But this may cause diagnostic problems for us, because if we have CIDP patients who already have severe axonal damage, we may not reach these criteria for demyelination in electrophysiology. So we're thinking more and more that early treatment may be important to prevent the axonal damage. Here on the right, you see one example showing data that axonal damage may indeed be a marker of clinical disability, where people with very severe axonal damage had a higher INCAT score than those with moderate to severe axonal damage.

