MS diagnosis: Uptake of 2024 criteria
This resource is only available for healthcare professionals outside of your region. Click below to browse other relevant learning materials.
How are the 2024 McDonald criteria shaping real-world practice in multiple sclerosis (MS)? Access a downloadable infographic and hear expert opinions on using the 2024 McDonald criteria for the diagnosis of MS. Leading MS experts Andrew Solomon (USA), Marcello Moccia (Italy), and Jiwon Oh (Canada) discuss their perspectives on topics including:
- Insights from a year of using the updated 2024 McDonald criteria in clinical practice, addressing special populations and misdiagnosis
- Oh’s clinical experience on the impact of the McDonald criteria revisions
- How the MS community is responding to the McDonald criteria revisions
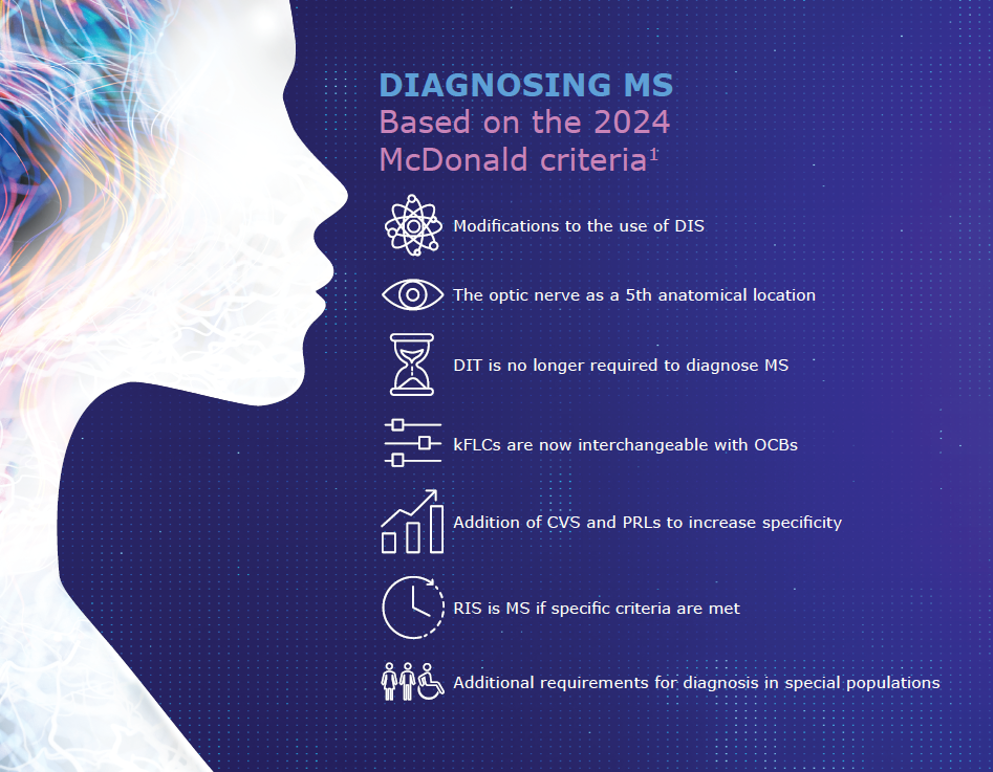
Infographic: Diagnosing MS based on the 2024 McDonald criteria
What are the fundamental principles of the 2024 McDonald criteria, how does the unified framework for MS diagnosis apply in clinical practice, and what are the recommendations for implementation? View the downloadable infographic below to find out more.
One year of updated McDonald criteria: What did we learn?
What impact have the revised McDonald criteria had on MS diagnosis 1 year after their introduction? Andrew Solomon (University of Vermont, Burlington, USA) and Marcello Moccia (University of Naples, Italy) discuss the most impactful updates in real-world practice, the implementation challenges clinicians may face, the role of emerging imaging markers, and key considerations for diagnosing MS in special populations.
Experiences, adoption, and practical considerations of the 2024 McDonald diagnostic criteria
What has the MS community response been to the revised 2024 McDonald diagnostic criteria? Have the revisions improved diagnosis in under-represented MS populations? Jiwon Oh (University of Toronto, Ontario, Canada) and Hashem Salloukh (Merck) consider the immediate impact on clinical practice, practical steps for implementation, community adoption, and challenges in broader application across low-resource settings.
Find additional expert insights and related content on the revised 2024 McDonald diagnostic criteria with Redefining MS: Diagnosis to patient goals.
Meet the experts
 Marcello Moccia, MD, PhD
Marcello Moccia, MD, PhD
Marcello Moccia is an Associate Professor at the University of Naples, Italy, and consultant neurologist and MS specialist at the Policlinico University Hospital. His clinical and research work focuses on MS, spanning immunology, neuroimaging, epidemiology, and translational applications, and he has authored more than 200 peer-reviewed publications.
Disclosures: Received a salary from University of Naples (Italy), Policlinico University Hospital (Naples, Italy), Neurology (AAN, US), and Multiple Sclerosis Journal (SAGE, UK); research grants from Campania Region (NeuroDiaTe CUP E65E24002260002), MUR PNRR Extended Partnership (MNESYS no. PE00000006, and DHEAL-COM no. PNC-E3-2022-23683267), the ECTRIMS-MAGNIMS, the UK MS Society, and Merck; honoraria from AbbVie, Biogen, BMS Celgene, Ipsen, Janssen, Merck, Novartis, Roche, and Sanofi-Genzyme.
 Jiwon Oh, MD, PhD
Jiwon Oh, MD, PhD
Jiwon Oh is a staff neurologist and Medical Director of the Barlo Multiple Sclerosis Program at St. Michael’s Hospital, University of Toronto, Canada. Her research focuses on developing advanced magnetic resonance imaging (MRI) techniques in the spinal cord and brain for use in clinical settings. She leads the MRI research programme at St. Michael’s Hospital, and is the principal investigator on numerous collaborative studies, including the Canadian Prospective Cohort Study to Understand Progression in MS (CanProCo), a national prospective cohort study to better understand progression in MS.
Disclosures: Personal compensation for consulting or speaking from Biogen, Eli Lilly, EMD Serono, Merck, Novartis, Roche, and Sanofi, and has received research support from Biogen, Merck, and Roche.
 Andrew Solomon, MD
Andrew Solomon, MD
Andrew Solomon is a Professor of Neurological Sciences and Division Chief of Multiple Sclerosis at the Larner College of Medicine at the University of Vermont, in Burlington, Vermont. He has been recognised nationally and internationally for clinical and research work centred on MS diagnosis, differential diagnosis, and misdiagnosis. His research focuses on the evaluation of novel imaging biomarkers for the diagnosis of MS.
Disclosures: Consulted for Octave Bioscience, Kiniksa Pharmaceuticals, and TG Therapeutics; served on the advisory board for TG Therapeutics, Horizon Therapeutics, Genentech/Roche, Sanofi, and Bristol Myers Squibb; non-promotional speaking for EMD Serono†, Merck, and Sanofi; site principal investigator for contract research for Sanofi, Actelion, Genentech/Roche, and Novartis; research funding from Bristol Myers Squibb.
*EMD Serono is the healthcare business of Merck in the US and Canada.
Developed by EPG Health, for Medthority. This content has been developed in collaboration with Merck. The materials shown in the presentations are intended for discussion purposes and must not be considered medical advice from a healthcare professional. EPG Health received funding from Merck in order to help provide its healthcare professional members with access to the highest quality medical and scientific information, education and associated relevant content. Any data on non-Merck products are based on publicly available information at the time of content update. Prescribing information may vary depending on local health authority approval in each country. Before prescribing any product, always refer to the SmPC or product information approved in your local country.
GL-NONNI-02568 | February 2026
of interest
are looking at
saved
next event