HSPC: Optimizing care
Exploring the HSPC treatment landscape
Available treatments for nmHSPC and mHSPC
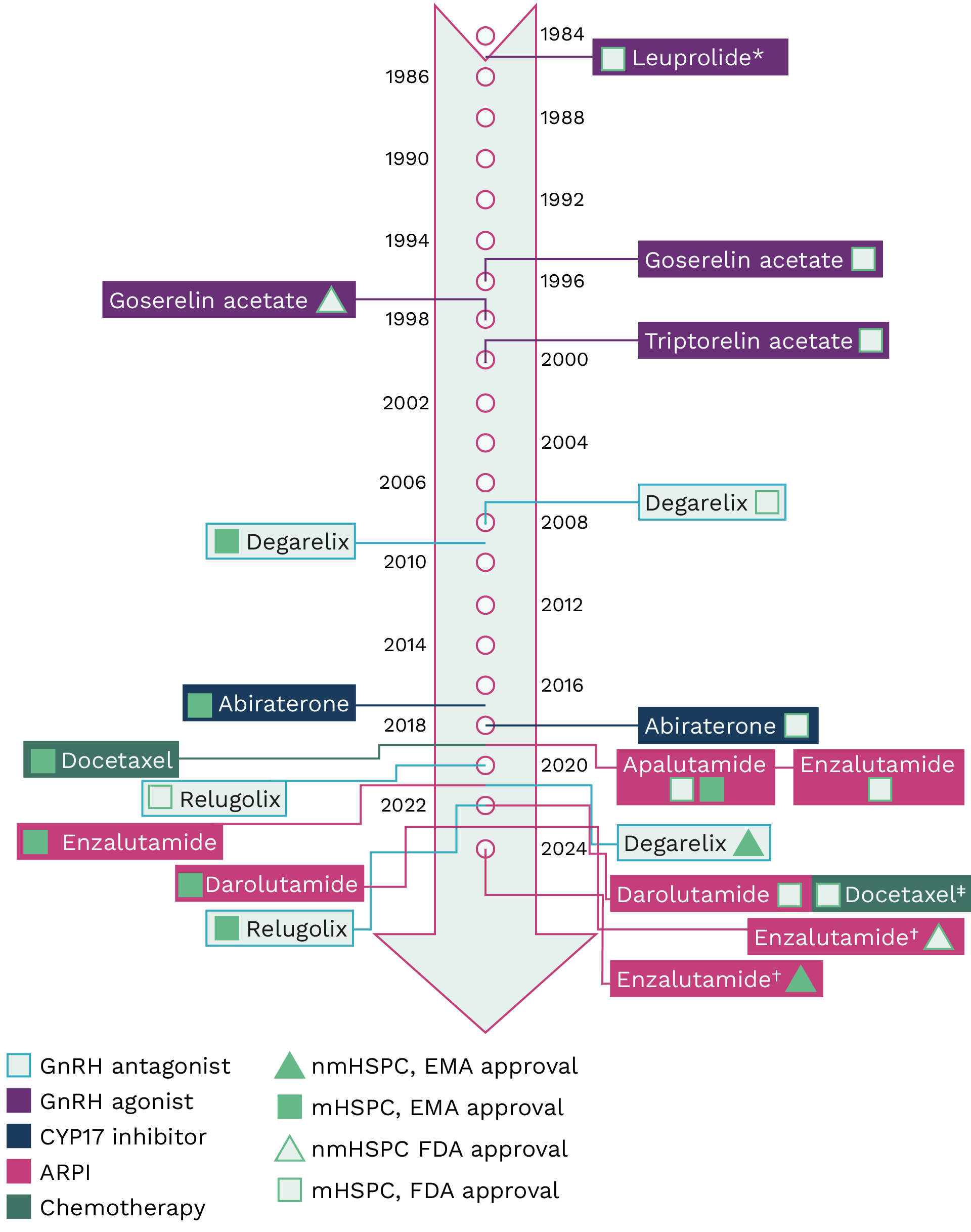
Treatment options for non-metastatic (nm) and metastatic (m) hormone-sensitive prostate cancer (HSPC) have expanded in recent years, as shown in Figure 1.1,2
Please note, Figure 1 has been created to demonstrate the expanding therapeutic area and should not be used to guide treatment decisions. Treatments have been listed based on initial approval dates, where available. Please refer to the prescribing information for each product for details on specific prescribing criteria, including patient criteria, requirements to prescribe as monotherapy or in combination with other agents (also known as treatment intensification), and safety considerations.
Access the EMA Summary of Product Characteristics (SmPC) and FDA Prescribing Information (PI) for enzalutamide. Adverse event reporting information can be found at the bottom of the page.
Figure 1. Timeline of treatment approvals for nmHSPC and mHSPC.3-27 *Leuprolide is also EMA approved for nmHSPC and mHSPC; information on approval date for this option is not available. †Enzalutamide is approved for the treatment of high-risk biochemically recurrent nmHSPC. Please refer to the enzalutamide prescribing information for full details.16 ‡Not licensed independently; recommended for use by the FDA within the darolutamide label, in combination with darolutamide, in 2022.25 ARPI, androgen receptor pathway inhibitor; EBRT, external beam radiation therapy; EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; GnRH, gonadotropin-releasing hormone; HSPC, hormone-sensitive prostate cancer; mHSPC, metastatic HSPC; nmHSPC, non-metastatic HSPC.
nmHSPC
The term nmHSPC is typically used to refer to a heterogeneous population of patients with localized prostate cancer that is androgen deprivation therapy (ADT)–naive.2,22
Initial treatment of people with localized prostate cancer is attempted with curative intent, or alternatively, patients are followed with active surveillance or “watchful waiting,” with life expectancy a key consideration2,28
Treatment options for nmHSPC include:
- Active surveillance28
- ADT, typically fixed duration1,2
- Radiotherapy, with or without ADT2,28
- Radical prostatectomy (RP)28
BCR treatment in nmHSPC
A tailored treatment strategy involving risk stratification is warranted for biochemical recurrence (BCR) in nmHSPC.29 However, a lack of consensus remains on whether to administer ADT for BCR, and optimal timing for ADT because of:
- Heterogeneity of BCR due to variation in disease course29
- Lack of consensus on prostate-specific antigen (PSA) thresholds for ADT initiation22
- Treatment-related adverse events and impact on quality of life2,22
Recent evidence suggests earlier and intensified therapy may be advantageous in some people with high-risk BCR, which has been variably defined across different guidelines and clinical trials22,26,30-33
Treatment options for BCR in nmHSPC include:
- Observation or active surveillance29
- External beam radiation therapy (EBRT) with or without ADT29
- Salvage ADT29
- Salvage RP29
- Brachytherapy or high-intensity focused ultrasound29
- Androgen receptor pathway inhibitor (ARPI), with or without ADT for BCR at high risk of metastasis16,17
- Other surgical or non-surgical options may be considered in select patients following ERBT29
In 2023 and 2024, the FDA and EMA approved the ARPI enzalutamide in patients with high-risk BCR:
- FDA indication: For the treatment of patients with nmHSPC and BCR at high risk of metastasis, with or without a gonadotropin-releasing hormone (GnRH) analog16
- EMA indication: As monotherapy or in combination with ADT for the treatment of adult men with high-risk BCR nmHSPC who are unsuitable for salvage radiotherapy17,34
mHSPC
ADT is increasingly considered suboptimal when used alone in mHSPC, and recent evidence has led to the adoption of the following combination therapies:1,32,35
- Doublet therapy with ADT plus either docetaxel chemotherapy or an ARPI36-39
- Triplet therapy with ADT plus docetaxel plus ARPI, typically used for high-volume metastatic disease38
In people with low-volume disease, treatments that combine ADT with docetaxel, abiraterone acetate, enzalutamide, apalutamide, darolutamide and/or radiotherapy to the primary tumor are now accepted as standard of care.1,36,38,40,41 For people with oligometastatic HSPC, metastasis-directed therapy can be considered as an option.36,42
Treatment guidelines for nmHSPC and mHSPC
Please note, the below information has been provided as an overview of treatment guidelines and does not constitute treatment guidance. Please refer to the full guidelines for a comprehensive summary of most current recommended monitoring and assessment criteria aligned to treatment recommendations. Before prescribing a treatment, always refer to your local summary of product characteristics or prescribing information.
nmHSPC
For localized or locally advanced nmHSPC, guidelines recommend that risk stratification, life expectancy, and symptomatology is used to inform treatment decisions26,28,30,43,44
- For low-risk disease, AS is recommended, according to EAU-EANM-ESTRO-ESUR-ISUP-SIOG 2024, ESMO 2020, and NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Version 3.2026 (Access date November, 7th, 2025)28,30,43
- NCCN Guidelines® recommend observation if life expectancy is <10 years30
- ESMO 2020 guidelines and NCCN Guidelines recommend RP or radiotherapy for low-risk disease not suitable for active surveillance, with NCCN Guidelines specifying this approach for patients with a life expectancy ≥10 years30,43
- For intermediate-risk disease, RP or radiotherapy, with or without ADT, can be used, according to EAU-EANM-ESTRO-ESUR-ISUP-SIOG 2024, ESMO 2020, and NCCN Guidelines28,30,43
- EAU-EANM-ESTRO-ESUR-ISUP-SIOG 2024 guidelines suggest AS may be appropriate for select patients with International Society of Urological Pathology (ISUP) grade group 2 disease28
- NCCN Guidelines recommend active surveillance for favorable intermediate-risk disease if life expectancy is >10 years30
- NCCN Guidelines recommend observation is also considered if life expectancy is 5–10 years and the disease is asymptomatic30
- For high-risk disease:
- NCCN Guidelines recommend ADT with radiotherapy or RP alone30
- EAU-EANM-ESTRO-ESUR-ISUP-SIOG 2024 guidelines recommend RP, extended pelvic lymph node dissection, or radiotherapy, with or without ADT28
- ESMO 2020 guidelines recommend either initial neoadjuvant ADT for 4–6 months with or without docetaxel followed by EBRT plus ADT, or RP plus pelvic lymphadenectomy43
- For very high–risk disease, a 2023 ESMO-published guideline update to the 2020 guidelines recommends radiotherapy plus ADT, abiraterone, and prednisone45
- In people with high- or very high–risk asymptomatic disease, and a life expectancy ≤5 years, NCCN Guidelines recommend observation, or radiotherapy with or without ADT30
If BCR occurs, treatment recommendations can be dependent on prior treatment lines and risk:
- Following RP, EAU-EANM-ESTRO-ESUR-ISUP-SIOG 2024, AUA-ASTRO-SUO 2023, ESMO 2020, and NCCN Guidelines recommend salvage radiotherapy with or without ADT*26,30,43,44
- The AUA-ASTRO-SUO 2023 guideline recommends this approach for people with high-risk features; in people without high-risk features, only radiation is recommended44
- Following radiotherapy, ESMO 2020 recommends local salvage therapy, or observation with delayed ADT43
- NCCN Guidelines recommend monitoring, or local secondary therapy with or without ADT or abiraterone if life expectancy is >5 years; ADT with or without abiraterone can also be considered30
- NCCN Guidelines recommend observation if life expectancy is ≤5 years following either RP or radiotherapy30
- For high-risk BCR, EAU-EANM-ESTRO-ESUR-ISUP-SIOG 2024 recommends enzalutamide with or without ADT†26
- In the event of low-risk second BCR, NCCN Guidelines recommend monitoring or ADT30
- In the event of high-risk second BCR, NCCN Guidelines recommend monitoring, or enzalutamide with or without leuprolide, or ADT alone†30
mHSPC
The mHSPC treatment landscape has evolved to include doublet and triplet therapy38
- In low-volume disease, EAU-EANM-ESTRO-ESUR-ISUP-SIOG 2024 guidelines recommend ADT plus radiotherapy for first presentation M1 disease. NCCN Guidelines recommend ADT with either ARPI, ARPI and EBRT, ARPI and docetaxel, or EBRT in people with synchronous metastases; in people with metachronous metastases, ADT is recommended with an ARPI26,30
- ESMO 2020 guidelines recommend ADT plus docetaxel or an ARPI, plus prostate radiotherapy for low-burden metastatic disease43
- For fit patients with M1 disease, ESTRO-EANM-ESUR-ISUP-SIOG 2024 recommend docetaxel plus ADT and a suitable ARPI, or ADT with abiraterone plus prednisone or a suitable ARPI26
- For high-volume disease (synchronous or metachronous), defined by the NCCN Guidelines as visceral metastasis or ≥4 bone lesions with ≥1 beyond the vertebral bodies and pelvis, the NCCN Guidelines recommend ADT plus ARPI, with or without docetaxel30
- For high-burden metastatic disease, the ESMO 2020 guideline recommends ADT plus docetaxel or an ARPI43
The 2023 ESMO guideline update also advises ADT alone for mHSPC should only be used in vulnerable men who cannot tolerate intensification (ADT in combination with ARPI, radiotherapy, and/or chemotherapy).45
While treatment options are expanding, the limited head-to-head comparison data on recently approved therapies mean the development of definitive treatment algorithms is challenging.38
Given the uncertain comparative efficacy of mHSPC combination therapies, management decisions should be personalized based on patient preferences and characteristics, and clinician expertise38
*NCCN Guidelines also recommend an option of radiotherapy plus ADT and abiraterone as category 2B.30
†Apalutamide plus ADT are also included as a category 2B recommendation for high-risk second BCR.
Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit–risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system. Adverse events should also be reported to Astellas. Please refer to the SmPC or PI approved in your local country for further information.
References
- López-Campos, 2021. Metastatic hormone-sensitive prostate cancer: How should it be treated? https://www.doi.org/10.5306/wjco.v12.i2.43
- Giunta, 2024. Pharmacological treatment landscape of non-metastatic hormone-sensitive prostate cancer: A narrative review on behalf of the meet-URO Group. https://www.doi.org/10.1016/j.critrevonc.2024.104534
- Camcevi, leuprorelin summary of opinion (initial authorisation), 2022. https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-opinion-camcevi_en.pdf
- Zoladex (goserelin acetate) approval letter, 1998. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/1998/19726s024ltr.pdf
- Trelstar (triptorelin pamoate) Prescribing Information, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/020715s049,021288s044,022437s024lbl.pdf
- Zoladex (goserelin acetate) Prescribing Information, 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020578s034,020578s035lbl.pdf
- Firmagon (degarelix) Prescribing Information, 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022201s009lbl.pdf
- Firmagon (degarelix) Summary of opinion (post authorisation), 2021. https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-firmagon-ii-39-g_en.pdf
- Firmagon (degarelix) Summary of Product Characteristics, https://www.ema.europa.eu/en/documents/product-information/firmagon-epar-product-information_en.pdf
- Orgovyx (relugolix) Prescribing Information, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214621s000lbl.pdf
- Zytiga (abiraterone acetate) Summary of opinion (post authorisation), 2017. https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-zytiga_en.pdf-0
- Orgovyx (relugolix) Summary of Product Characteristics, https://www.ema.europa.eu/en/documents/product-information/orgovyx-epar-product-information_en.pdf
- U.S. Food and Drug Administration, 2019. FDA approves apalutamide for metastatic castration-sensitive prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-apalutamide-metastatic-castration-sensitive-prostate-cancer
- Erleada (apalutamide) Summary of opinion (post authorisation), 2019. https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-erleada-ii-01_en.pdf
- U.S. Food and Drug Administration, 2023. FDA approves enzalutamide for non-metastatic castration-sensitive prostate cancer with biochemical recurrence. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-enzalutamide-non-metastatic-castration-sensitive-prostate-cancer-biochemical-recurrence
- Xtandi (enzalutamide) Prescribing Information, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/213674s011,203415s023lbl.pdf
- Astellas, 2024. Astellas’ XTANDI (Enzalutamide) granted European Commission approval for use in additional recurrent early prostate cancer treatment setting. https://www.astellas.com/en/news/29146
- 2021. Astellas' XTANDI (enzalutamide) Approved by European Commission for Men with Metastatic Hormone-Sensitive Prostate Cancer. https://newsroom.astellas.us/2021-05-04-Astellas-XTANDI-TM-enzalutamide-Approved-by-European-Commission-for-Men-with-Metastatic-Hormone-Sensitive-Prostate-Cancer
- Oncology Nursing News, 2025. Darolutamide receives FDA approval for mCSPC. https://www.oncnursingnews.com/view/darolutamide-receives-fda-approval-for-mcspc
- Nubeqa (darolutamide) Summary of opinion (post authorisation), 2023. https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-opinion-nubeqa-ii-09_en.pdf
- Azad, 2025. Combination therapies in locally advanced and metastatic hormone-sensitive prostate cancer. https://www.doi.org/10.1016/j.eururo.2025.01.010
- Karim, 2025. Early versus delayed androgen deprivation therapy for biochemical recurrence after local curative treatment in non-metastatic hormone-sensitive prostate cancer: A systematic review of the literature. https://www.doi.org/10.3390/cancers17020215
- Leith, 2022. Impact of next-generation hormonal agents on treatment patterns among patients with metastatic hormone-sensitive prostate cancer: A real-world study from the United States, five European countries and Japan. https://www.doi.org/10.1186/s12894-022-00979-9
- Chwalisz, 2023. Clinical development of the GnRH agonist leuprolide acetate depot. https://www.doi.org/10.1016/j.xfre.2022.11.011
- Nubeqa (darolutamide) Prescribing Information, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212099s002lbl.pdf
- Tilki, 2024. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer. Part II—2024 update: Treatment of relapsing and metastatic prostate cancer. https://www.doi.org/10.1016/j.eururo.2024.04.010
- Morgan, 2024. Salvage therapy for prostate cancer: AUA/ASTRO/SUO guideline part I: Introduction and treatment decision-making at the time of suspected biochemical recurrence after radical prostatectomy. https://www.doi.org/10.1097/ju.0000000000003892
- Cornford, 2024. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer—2024 update. Part I: Screening, diagnosis, and local treatment with curative intent. https://www.doi.org/10.1016/j.eururo.2024.03.027
- Shore, 2024. Biochemical recurrence in patients with prostate cancer after primary definitive therapy: Treatment based on risk stratification. https://www.doi.org/10.1038/s41391-023-00712-z
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer V.3.2026. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Access date November 7, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Virgo, 2023. Initial management of noncastrate advanced, recurrent, or metastatic prostate cancer: ASCO guideline update. https://www.doi.org/10.1200/jco.23.00155
- Lowrance, 2023. Updates to advanced prostate cancer: AUA/SUO guideline (2023). https://www.doi.org/10.1097/ju.0000000000003452
- Lowrance, 2021. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. https://www.doi.org/10.1097/ju.0000000000001375
- Xtandi (enzalutamide) Summary of Product Characteristics, https://www.ema.europa.eu/en/documents/product-information/xtandi-epar-product-information_en.pdf
- Leith, 2022. Real-world treatment patterns in metastatic castration-resistant prostate cancer across Europe (France, Germany, Italy, Spain, and the United Kingdom) and Japan. https://www.doi.org/10.1007/s12325-022-02073-w
- Cattrini, 2019. Current treatment options for metastatic hormone-sensitive prostate cancer. https://www.doi.org/10.3390/cancers11091355
- Ong, 2021. Current treatment options for newly diagnosed metastatic hormone-sensitive prostate cancer-a narrative review. https://www.doi.org/10.21037/tau-20-1118
- Smani, 2025. Advances in current treatment paradigms for metastatic hormone-sensitive prostate cancer. https://www.doi.org/10.3390/jcm14082565
- U.S. Food and Drug Administration, 2025. FDA approves darolutamide for metastatic castration-sensitive prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-darolutamide-metastatic-castration-sensitive-prostate-cancer
- Nubeqa (darolutamide) Prescribing Information, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/212099s008lbl.pdf
- Saad, 2024. Darolutamide in combination with androgen-deprivation therapy in patients with metastatic hormone-sensitive prostate cancer from the phase III ARANOTE trial. https://www.doi.org/10.1200/jco-24-01798
- Fiorica, 2025. Metastasis-directed therapy in oligometastatic prostate cancer: Biological rationale and systematic review of published data. https://www.mdpi.com/2072-6694/17/8/1256
- Parker, 2020. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. https://www.doi.org/10.1016/j.annonc.2020.06.011
- Morgan, 2024. Salvage therapy for prostate cancer: AUA/ASTRO/SUO guideline part II: Treatment delivery for non-metastatic biochemical recurrence after primary radical prostatectomy. https://www.doi.org/10.1097/ju.0000000000003891
- Fizazi and Gillessen, 2023. Updated treatment recommendations for prostate cancer from the ESMO clinical practice guideline considering treatment intensification and use of novel systemic agents. https://www.doi.org/10.1016/j.annonc.2023.02.015
MA-MM-16218, November 2025.
Elevating HSPC care in the MDT
There is evidence that the use of multidisciplinary teams (MDTs) in hormone-sensitive prostate cancer (HSPC) can lower physician bias, promote racial equality, and lead to:1
- Improved overall survival2
- Increased treatment plan alteration2
- Increased cross-referral rates1,2
- Implementation of innovative treatment options3
- Increased clinical trial inclusion rates1,2
However, inconsistencies in MDT engagement across specialists, along with the challenge of maintaining deep familiarity with a wide breadth of management approaches, can create obstacles to MDT care.1,2
An MDT in the prostate cancer setting may include:1
- Urologists1
- Radiation oncologists1
- Medical oncologists1
- Pathologists1
- Nuclear medicine specialists1
- Primary care physicians1
- Interventional radiologists1
- Palliative and supportive care practitioners1
- Nurses1
- Pharmacists1
- Patient advocates1
- Cardio-oncologists4
- Genetic counselors5
Multidisciplinary involvement in HSPC care and the patient experience
“In this MDT, we have to have this cross collaboration to optimize the outcome and manage the disease.” Axel Merseburger (University Hospital Schleswig-Holstein, Lübeck, Germany) draws from his clinical experience in HSPC, discussing the importance of communication between MDT members, shared decision-making with patient involvement, and the exchange of knowledge and expertise across different specialties. View transcript.
To relieve patients of the responsibility of identifying specialists and coordinating care, urologists are encouraged to establish referral networks and build relationships with other specialists.6 The rise in cardiovascular disease in men with HSPC has also highlighted the importance of integrating cardio-oncologists into the MDT.4,6 Identifying an optimal method of communication within the MDT, for example telemedicine or other digital platforms, is essential to improve coordination.6
Key factors in multidisciplinary team treatment decisions
“There are multiple factors – very important factors – that influence the management decision within the MDT.” Axel Merseburger considers who is involved in the MDT, individual patient characteristics, and the importance of MDT members’ awareness of patient preferences in HSPC care. View transcript.
Before making treatment decisions for HSPC within the MDT, various factors need to be considered:7
- Mechanism of action
- Route of administration
- Duration of treatment
- Impact on quality of life
- Toxicity profile
- Patient preferences, comorbidities, and individual characteristics
Physician–patient shared decision-making can improve patient outcomes, satisfaction, engagement, management plans, and adherence1,2,8
In cases where there are multiple management options with limited data, shared decision-making with patients and communicating the benefits and risks of each option is essential.1,5
It is also important to consider cost effectiveness, availability of treatments in your local region, and the impact of differences in rural/urban or community/academic settings.5,7 While MDTs can address possible disparities via shared knowledge and resources, rural or community settings can also be barriers to the MDT.5
Meet the expert
 Axel Merseburger, MD, PhD
Axel Merseburger, MD, PhD
Axel Stuart Merseburger is a professor and chairman at the Department of Urology at the University Hospital Schleswig-Holstein in Lübeck, Germany. With over 21 years in clinical practice, he is highly regarded for his extensive experience as a urologist, his skills in robotic surgery, and his focus on prostate cancer. His dedication to research, innovative treatments, patient care, and advancing the field of urology makes him a valuable asset to the medical community.
Disclosures: Lecture/speaker honoraria, consultancy, and/or research and clinical trials fees from Ambu, Amgen, Apogepha, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, EUSAPharma, Farco, Ferring, Hexal, Ipsen, Janssen, MedUpdate, Merck Serono, MSD, Novartis, Pfizer, Recordati, Roche, Sandoz, Takeda, and Teva.
References
- Efstathiou, 2024. Novel hormone therapy and coordination of care in high-risk biochemically recurrent prostate cancer. https://www.doi.org/10.1016/j.ctrv.2023.102630
- Zhu, 2021. Dynamic multidisciplinary team discussions can improve the prognosis of metastatic castration-resistant prostate cancer patients. https://www.doi.org/10.1002/pros.24167
- Creemers, 2023. Role of multidisciplinary team meetings in implementation of chemohormonal therapy in metastatic prostate cancer in daily practice. https://www.doi.org/10.1038/s41391-022-00556-z
- Cirne, 2025. Principles of optimal multidisciplinary management of prostate cancer in clinical practice. https://www.doi.org/10.1186/s40959-025-00322-9
- Shore, 2022. Addressing challenges and controversies in the management of prostate cancer with multidisciplinary teams. https://www.doi.org/10.1007/s11523-022-00925-7
- Merseburger, 2024. Cardiovascular disease risk assessment and multidisciplinary care in prostate cancer treatment with ADT: recommendations from the APMA PCCV expert network. https://www.doi.org/10.1007/s00345-024-04852-2
- Cattrini, 2019. Current treatment options for metastatic hormone-sensitive prostate cancer. https://www.doi.org/10.3390/cancers11091355
- de Angst, 2019. Should we involve patients more actively? Perspectives of the multidisciplinary team on shared decision-making for older patients with metastatic castration-resistant prostate cancer. https://www.doi.org/10.1016/j.jgo.2018.12.003
MA-MM-16220, November 2025.
HSPC: Emerging evidence and looking forward
The evolution of hormone-sensitive prostate cancer (HSPC) management requires multidisciplinary and precision-based approaches to improve patient outcomes1
Access the EMA Summary of Product Characteristics (SmPC) and FDA Prescribing Information (PI) for enzalutamide. Adverse event reporting information can be found at the bottom of the page.
What can we expect from the future of HSPC research in the clinical setting? Axel Merseburger (University Hospital Schleswig-Holstein, Lübeck, Germany) reviews the available treatment options, quality of life (QoL) data, and investigational screening and treatment methods for non-metastatic HSPC (nmHSPC) and metastatic HSPC (mHSPC). View transcript.
Assessing QoL in HSPC
Significant heterogeneity and bias in PROM and health-related QoL assessment in clinical trials highlight the need for standardization in both real-world practice and research to improve reliability and enhance patient care2,3
While there are numerous available management options for HSPC, metastatic prostate cancer cases are rising, more men are presenting with advanced-stage disease, and both HSPC and its treatment options can significantly impact QoL.1,4
Assessment of QoL is considered increasingly important, and further integration of patient-reported outcome or experience measures (PROMs or PREMs) into clinical trials is needed to improve clinical practice effectively.2,4
A 2024 meta-analysis of PROMs and PREMs in radical prostatectomy and radiotherapy clinical trials found the treatments affected urinary, bowel, and sexual functions with varying severity.2
Baseline cognitive impairment is experienced by many men with prostate cancer and is thought to be affected by treatment.5 However, cognitive status is often not assessed at baseline, making it difficult to determine the extent of treatment-related decline.6 In addition, patients with cognitive impairment are not sufficiently represented in studies, and it can be difficult to diagnose due to its complexity.5
Geographical disparities in HSPC care
Real-world data from five countries between 2018 and 2020 found 76% of men with mHSPC did not receive treatment aligned to clinical guidelines7
Additionally, clinical trials in low-resource settings, such as Sub-Saharan Africa, are very limited; new approaches are needed that differ from high- and upper–middle-income countries.8 For example, while prostate-specific antigen (PSA) cut-off values or serum levels and age-specific screening are conventional in many countries, they are not established in Sub-Saharan Africa.8
Therefore, further research is needed to develop recommendations and effective measures across different populations.8
Future advances in HSPC care
There is still a need for further investigation into screening methods, novel treatments, and impact on QoL across different populations.1,4
Advances in imaging techniques, such as multi-parametric magnetic resonance imaging (mpMRI), micro-ultrasound (microUS), prostate-specific membrane antigen (PSMA)-positron emission tomography (PET), and computed tomography imaging, have improved the sensitivity and specificity of metastatic disease detection. Further evidence, especially from randomized controlled trials, is required to clarify the most effective role and timing of these imaging modalities in prostate cancer care.1,9,10
Further biomarkers to stratify patients with prostate cancer, in particular in the nmHSPC setting, are also needed, and while a rapid PSA test has been developed to reduce dependency on laboratory quality control, it is not yet commercially available.8,11 Alternative radioligands to PSMA are also being investigated but are not widely used in clinical practice.1
Numerous emerging treatments are being investigated for potential use in nmHSPC and mHSPC, including targeted therapies such as radioligand therapy and poly (ADP-ribose) polymerase (PARP) inhibitors.12 Treatment decision-making that is informed by biomarkers is currently under investigation.1
Meet the expert
Axel Merseburger, MD, PhD
Axel Stuart Merseburger is a professor and chairman at the Department of Urology at the University Hospital Schleswig-Holstein in Lübeck, Germany. With over 21 years in clinical practice, he is highly regarded for his extensive experience as a urologist, his skills in robotic surgery, and his focus on prostate cancer. His dedication to research, innovative treatments, patient care, and advancing the field of urology makes him a valuable asset to the medical community.
Disclosures: Lecture/speaker honoraria, consultancy, and/or research and clinical trials fees from Ambu, Amgen, Apogepha, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, EUSAPharma, Farco, Ferring, Hexal, Ipsen, Janssen, MedUpdate, Merck Serono, MSD, Novartis, Pfizer, Recordati, Roche, Sandoz, Takeda, and Teva.
Reporting suspected adverse reactions after authorization of the medicinal product is important. It allows continued monitoring of the benefit–risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system. Adverse events should also be reported to Astellas. Please refer to the SmPC or PI approved in your local country for further information.
References
- Smani, 2025. Advances in current treatment paradigms for metastatic hormone-sensitive prostate cancer. https://www.doi.org/10.3390/jcm14082565
- Alberti, 2024. Patient-reported outcome measures and experience measures after active surveillance versus radiation therapy versus radical prostatectomy for prostate cancer: A systematic review of prospective comparative studies. https://www.doi.org/10.1016/j.euo.2024.05.008
- Osanto, 2024. Health-related quality of life outcomes in randomized controlled trials in metastatic hormone-sensitive prostate cancer: a systematic review. https://www.doi.org/10.1016/j.eclinm.2024.102914
- Briggs, 2022. Optimal assessment of quality of life for patients with prostate cancer. https://www.doi.org/10.1177/17588359221141306
- Cowan, 2024. Treatment-related cognitive impairment in patients with prostate cancer: Patients' real-world insights for optimizing outcomes. https://www.doi.org/10.1007/s12325-023-02721-9
- Wefel, 2022. Assessment and management of cognitive function in patients with prostate cancer treated with second-generation androgen receptor pathway inhibitors. https://www.doi.org/10.1007/s40263-022-00913-5
- Goebell, 2024. Real-world treatment of metastatic hormone-sensitive prostate cancer in the USA, Europe and Asia. https://www.doi.org/10.2217/fon-2023-0814
- Bosland, 2023. Potential new approaches for prostate cancer management in resource-limited countries in Africa. https://www.doi.org/10.5334/aogh.3994
- Karpinski, 2024. Combining PSMA-PET and PROMISE to re-define disease stage and risk in patients with prostate cancer: a multicentre retrospective study. https://www.doi.org/10.1016/s1470-2045(24)00326-7
- Albers and Kinnaird, 2024. Advanced imaging for localized prostate cancer. https://www.mdpi.com/2072-6694/16/20/3490
- Giunta, 2024. Pharmacological treatment landscape of non-metastatic hormone-sensitive prostate cancer: A narrative review on behalf of the meet-URO Group. https://www.doi.org/10.1016/j.critrevonc.2024.104534
- Bourlon, 2024. Development of PARP inhibitors in advanced prostate cancer. https://www.doi.org/10.1177/17588359231221337
MA-MM-16221, November 2025.
This educational program, including expert panel and interviews, is sponsored by Astellas. This content includes information about investigational compounds that may be approved by regulatory agencies for specific indications, have been submitted to regulatory agencies for approval for specific indications, or are being studied in clinical trials and do not yet have a regulatory approval or authorization for a specific indication. Information about other potential uses of these products or investigational compounds is intended only for the purposes of medical education and is aimed at increasing the scientific knowledge of healthcare professionals (HCPs), to enhance medical practice and improve patient outcomes and should not be interpreted as intent to promote unapproved uses. Indications and availability of products discussed in this educational meeting may vary in different countries. Please refer to the local summary of product characteristics/prescribing information for details. Astellas prohibits the promotion of unapproved uses and complies with all applicable laws, regulations, and company policies. In expert interviews, podcasts, and panel discussions, the views, thoughts, and opinions expressed belong solely to the speaker(s) and are subject to change without notice. This content is intended for HCPs only. Non-HCPs should not view this educational program and should exit the program as soon as possible.